Genomics is the study of DNA and has been incorporated into dairy genetic evaluations in Ireland since Spring 2009. Genomics will accurately identify the genetically superior candidate parents of the next generation.
How does it work?
The first step in genomic selection is to establish a reference population. The reference population is a large genotyped population of animals with accurate performance information such as milk yield or fertility. The associations between the DNA and performance measures are then derived from this population. Several thousand animals are required to form a good reference population; presently there are over 8,000 informative animals within the Irish Holstein-Friesian reference population. Increasing the size of the reference population is essential to ensure genomic prediction estimates are accurate. The average reliability of genomic proofs of young animals is now 63%. A recent validation exercise revealed the accuracy of genomic evaluations is 16%-35% more accurate than evaluations based solely on parental average. New research clearly shows that there is a further benefit if genotyped cows are also included in the reference population; the accuracy improves a further 5–10% over just using information on sires (Table 1).
Value of genotyping females in the herd.
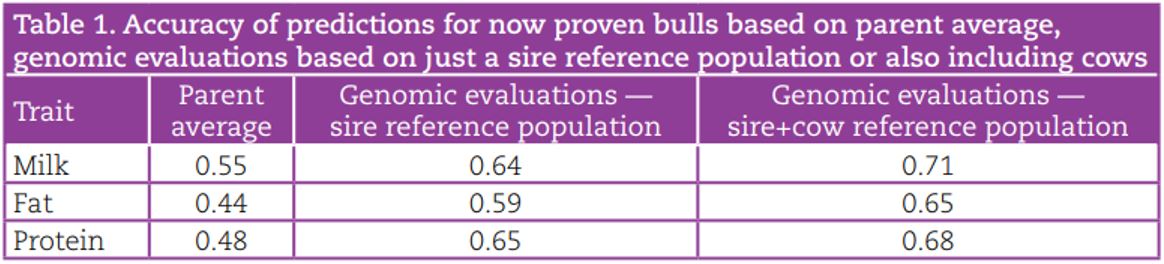
The current cost of genotyping all female calves in a herd is €22/head (incl. VAT). This is considerably cheaper than overseas, where genotyping costs range from €31 – €90. The cost of genotyping female dairy calves can be recouped through better selection of herd replacements. This is illustrated in the Figure 1, where the breakeven genotyping cost at a 21% replacement rate assuming 80% of females genotyped are retained is €49, more than double the actual cost. For example, a herd that keeps 80% of heifer calves as replacements and has a replacement rate of 21% has an expected net benefit of genotyping of €33 per heifer retained. Genomic information can also be used to confirm parentage, identify lethal/major genes, estimate inbreeding and predict the breed composition of an animal.
Minimising risk
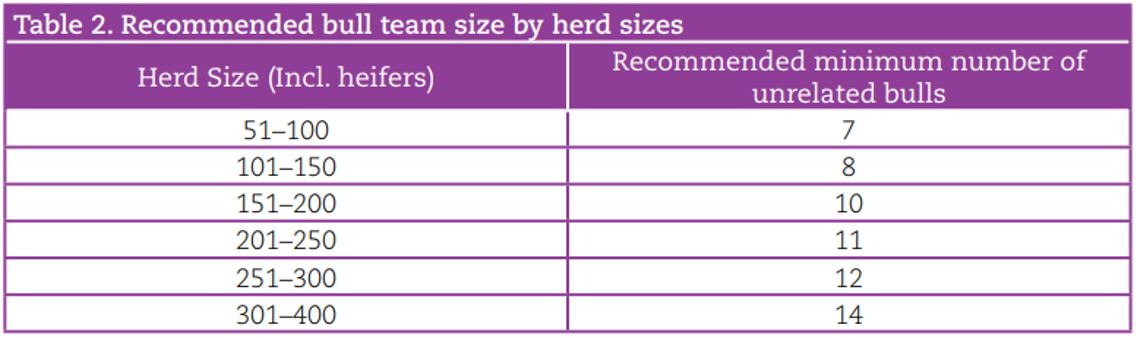
Despite the recent increases in reliability with genomic selection, it is important to acknowledge that the EBI of an individual animal can still change over time as more information accumulates. To minimise the potential risk of using young genomic bulls, a bull team should be used with the minimum team size being dependent on the herd size (Table 2); this achieves a bull team reliability of 95%. For example, in a 100-cow herd, equal usage of eight unrelated genomic sires is recommended to mitigate proof fluctuation. While using daughter proven bulls instead of genomic bulls can reduce risk on an individual bull level, it reduces genetic gain. This was seen when comparing seven high EBI genomic bulls and seven high EBI daughter proven bulls from the 2011 active bull lists with their 2017 EBI. Results showed that, on average, the team of seven genomic bulls were €52 ahead of the daughter proven bull team in 2011 to 2017.
Conclusions
Genomic selection is accelerating the rate of genetic gain in EBI through the more accurate identification of genetically elite males and females. It is, however, important to minimise risk associated with genomic selection by using large bull teams.
